2024
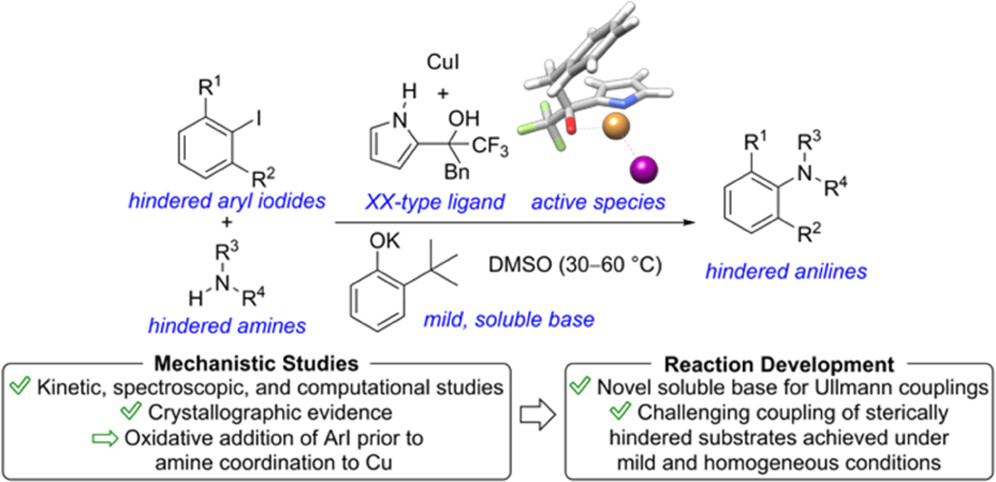
de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).
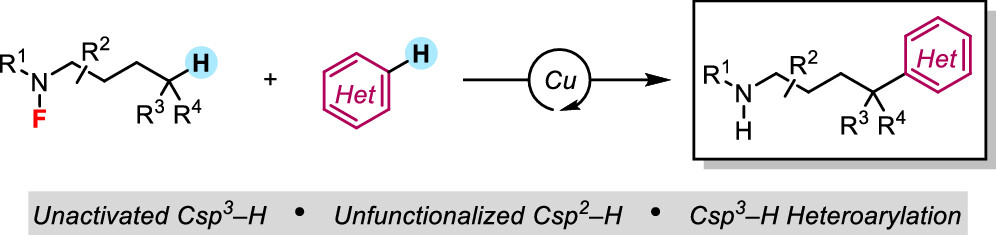
Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].
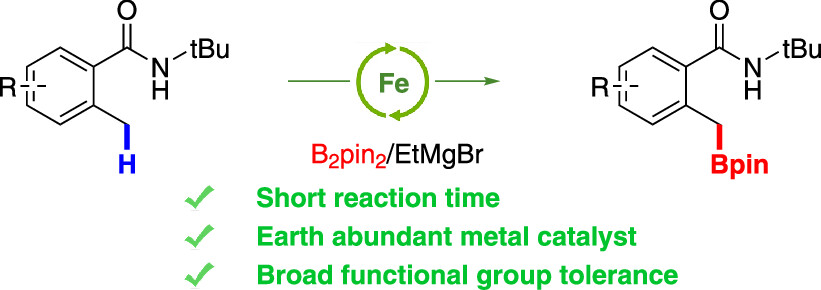
Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].
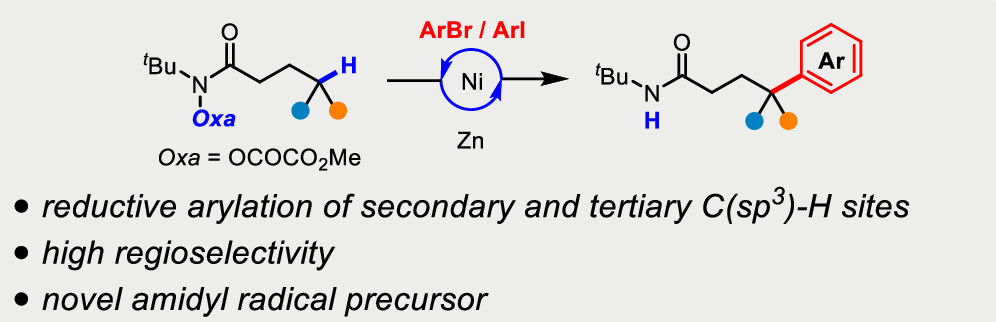
Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].
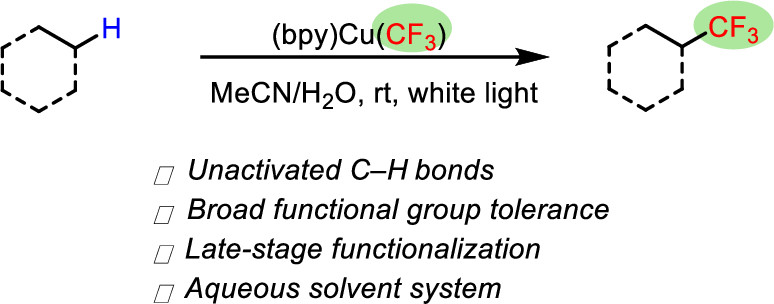
He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].
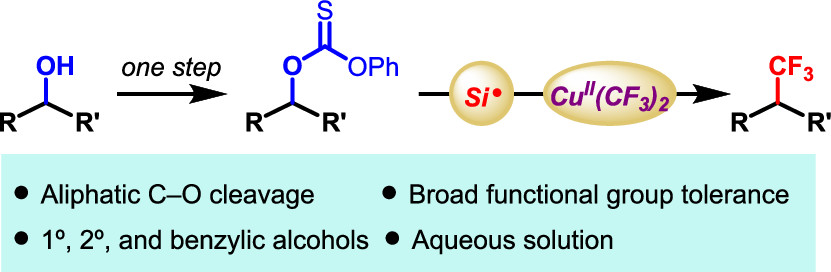
Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].
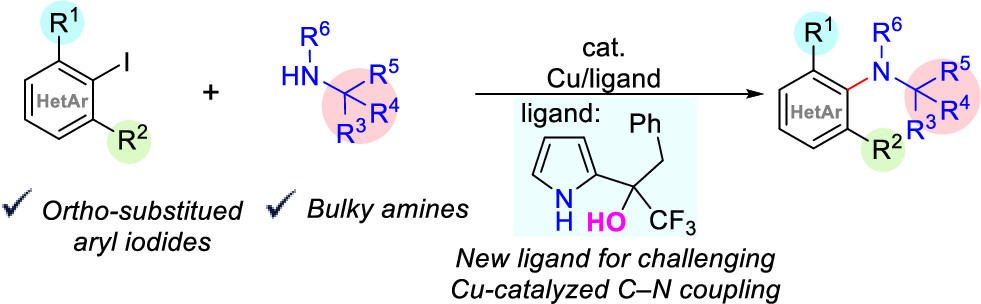
Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).
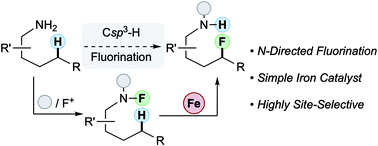
Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).
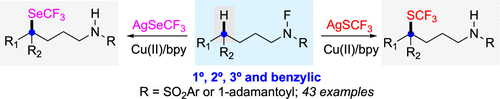
Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
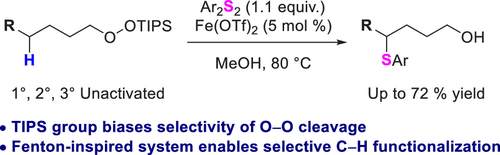
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).
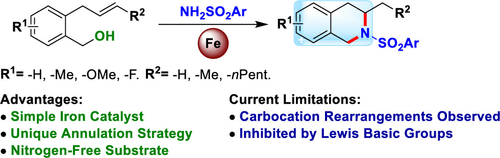
Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]
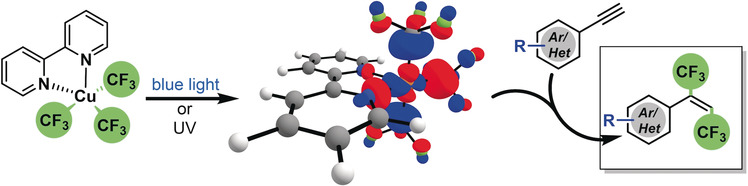
Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]
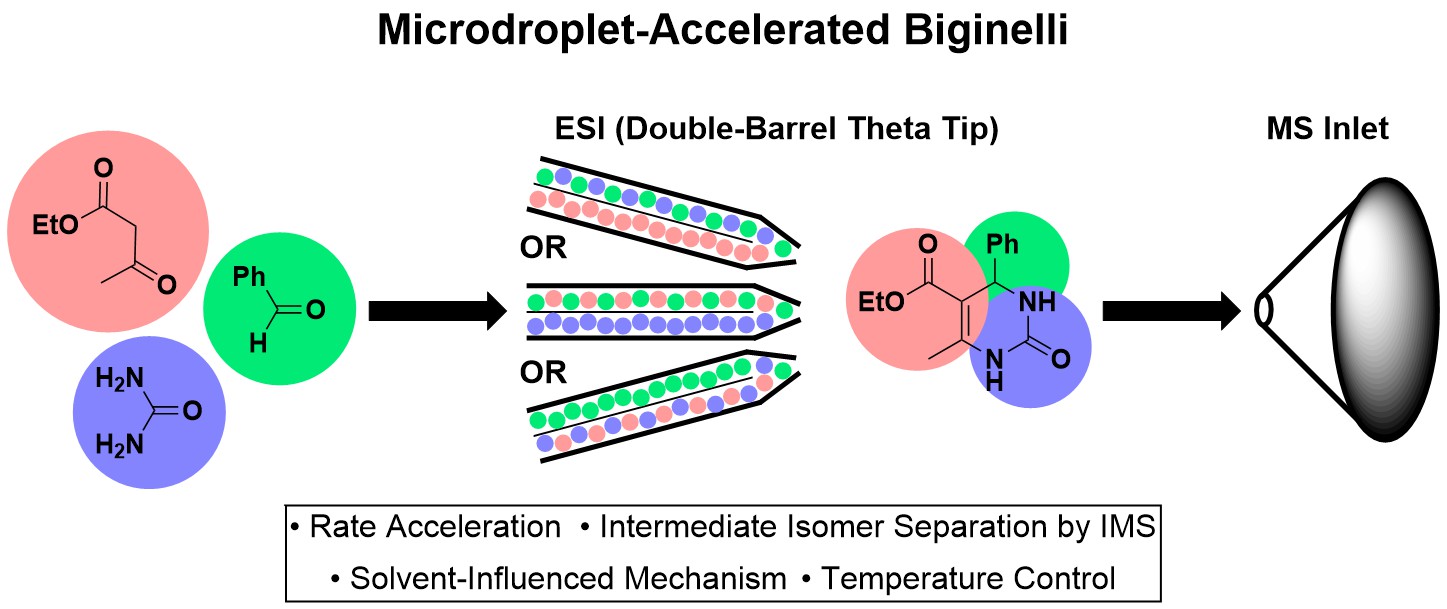
Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].
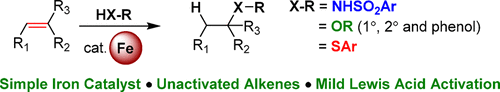
Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]
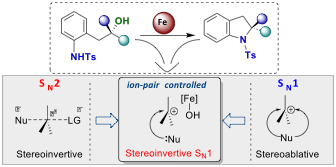
Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]
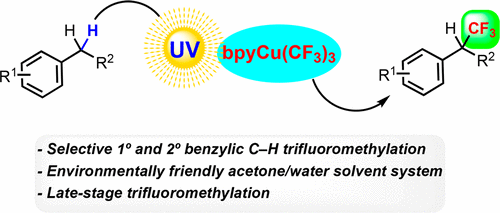
Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!
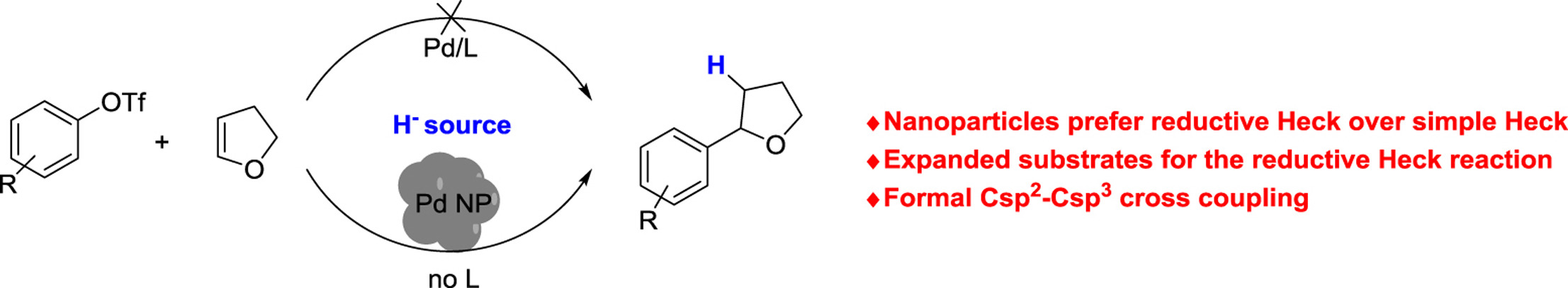
Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.
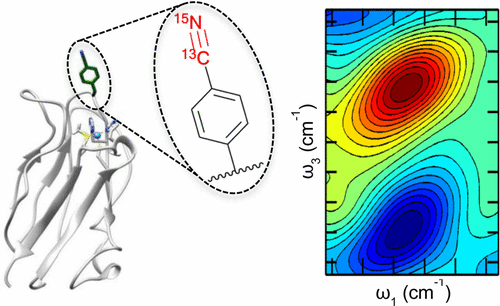
Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]
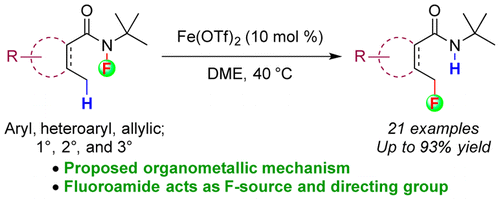
Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]
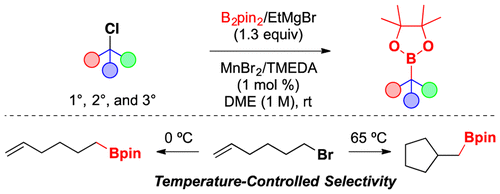
Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]
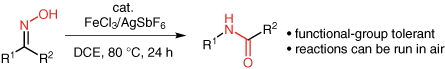
Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.
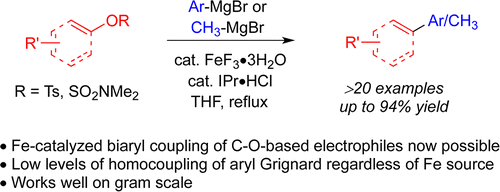
Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]
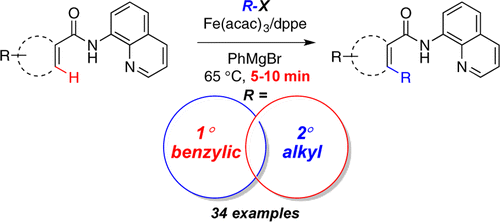
Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]
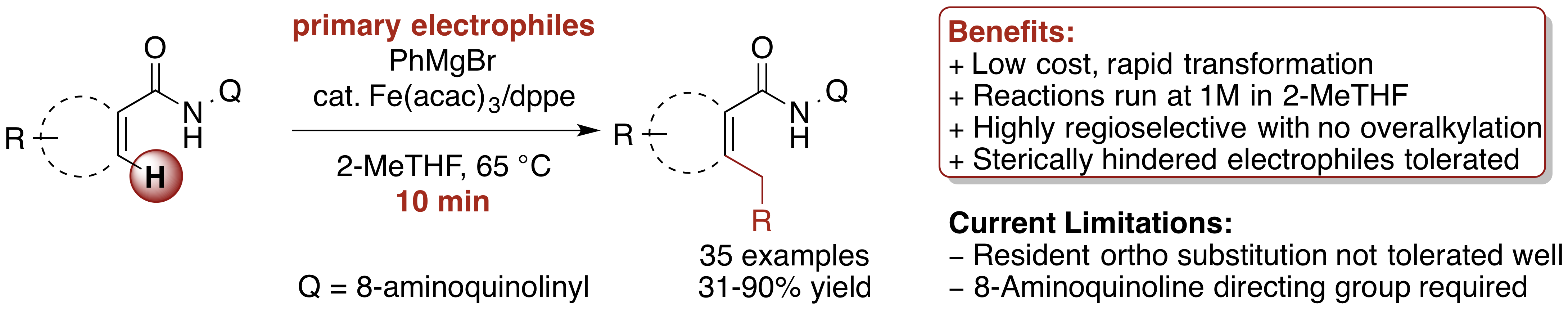
Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.
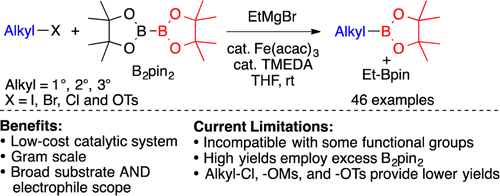
Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]
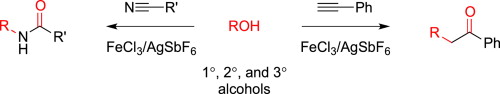
Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.
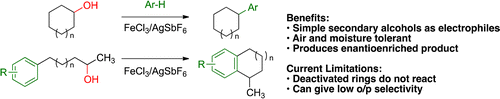
Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]
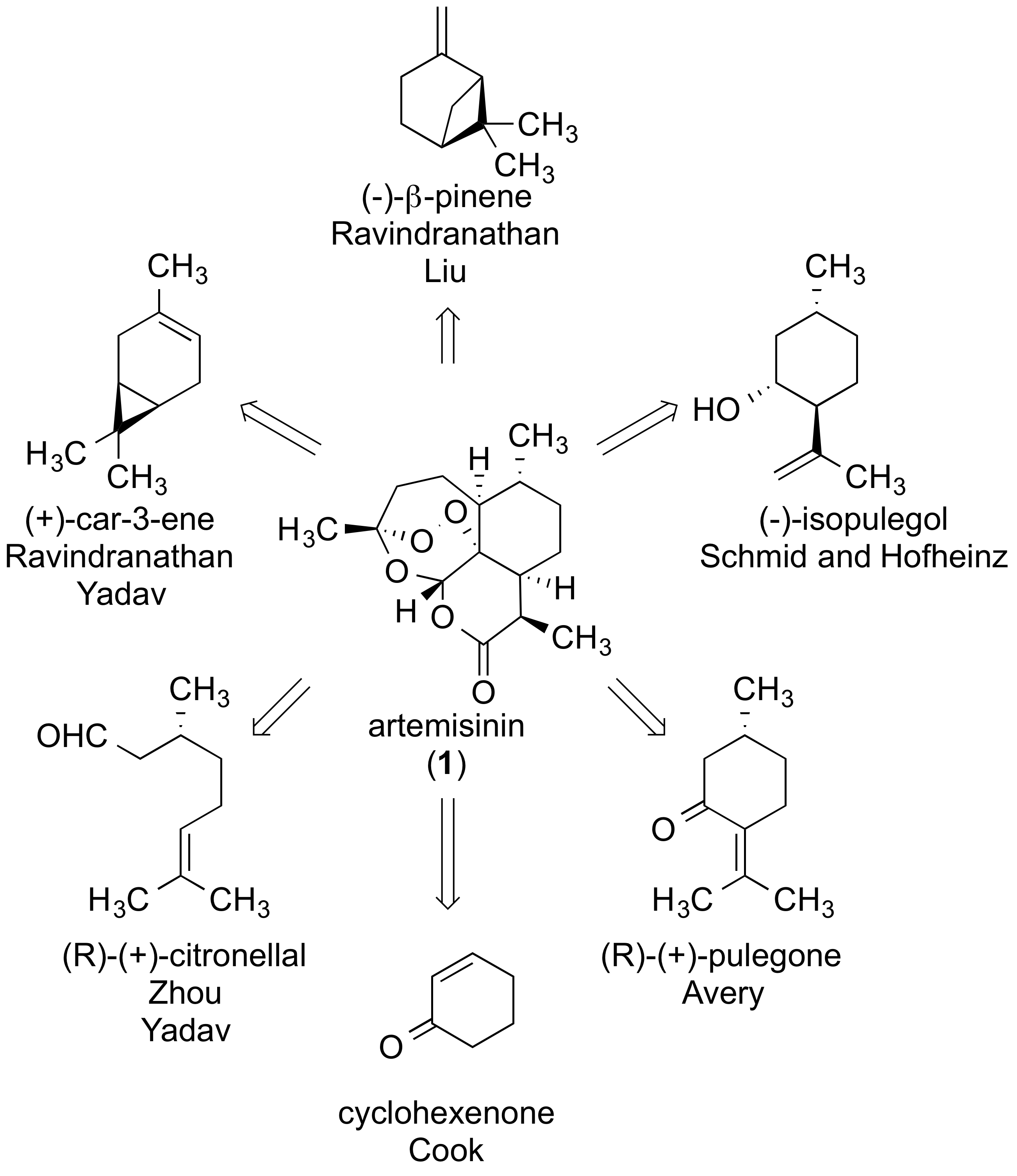
Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.
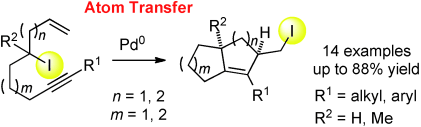
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]
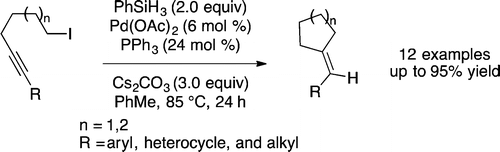
Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.
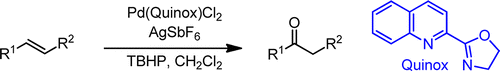
DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]
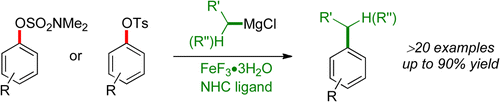
Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
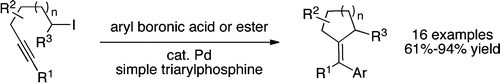
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]
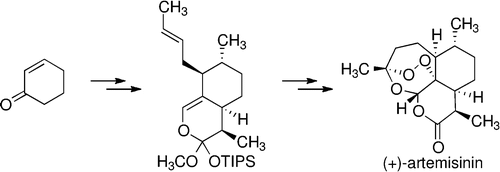
Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]
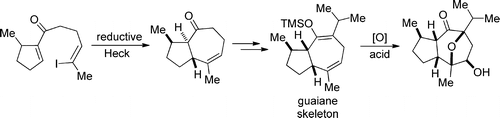
Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]
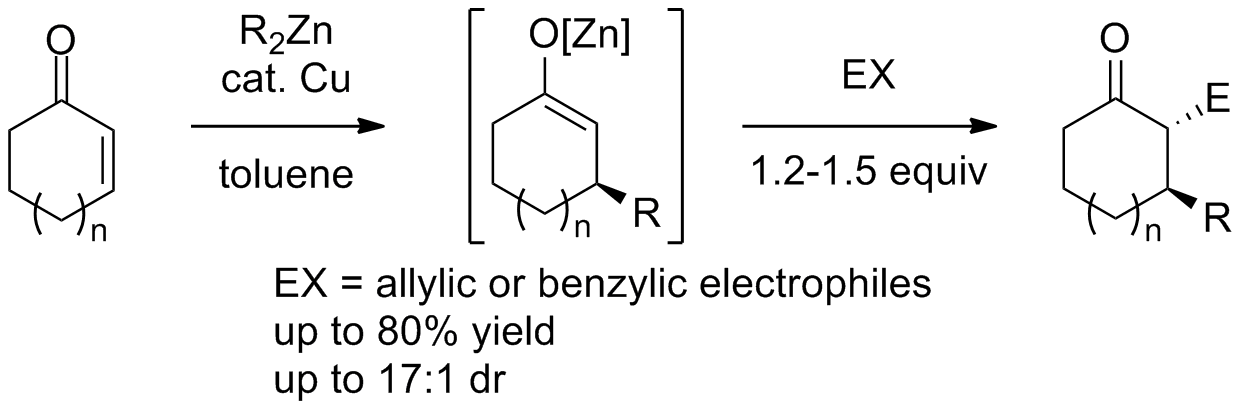
Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
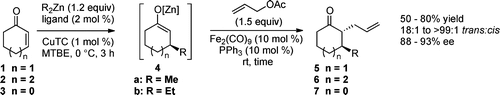
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2023

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
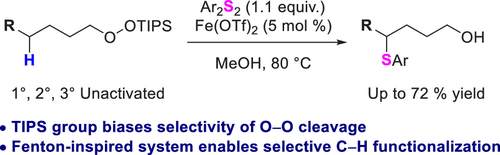
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
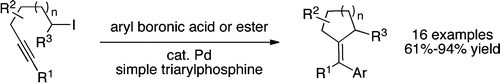
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
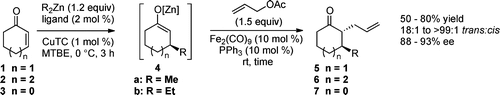
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2022

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
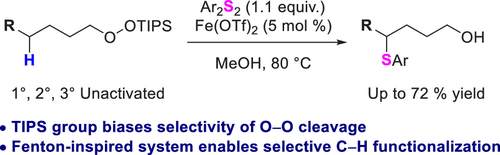
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
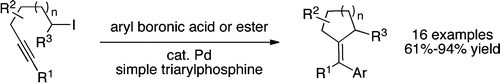
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
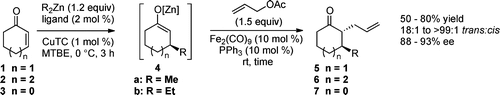
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2021

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
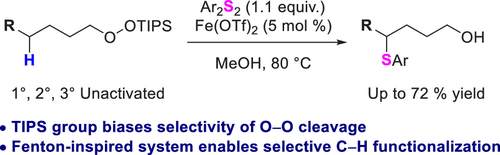
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
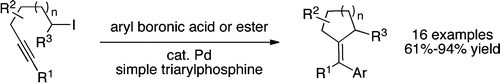
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
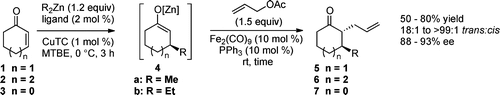
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2020

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
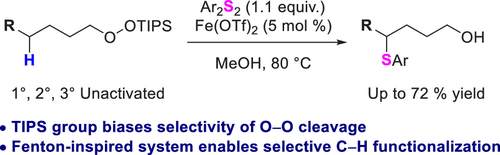
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
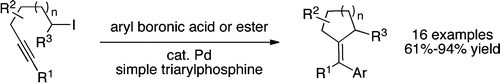
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
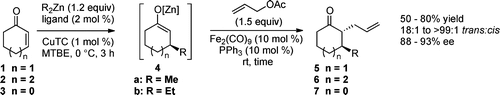
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2019

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
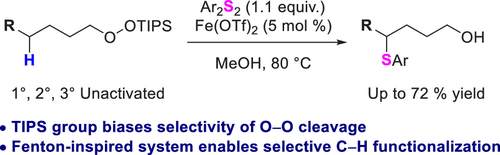
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
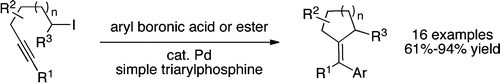
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
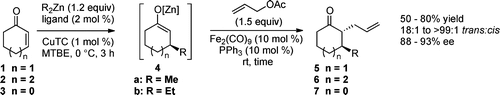
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2018

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
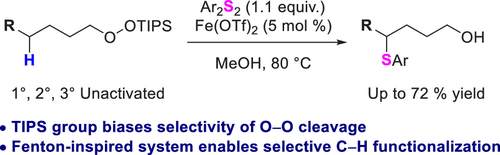
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
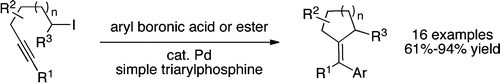
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
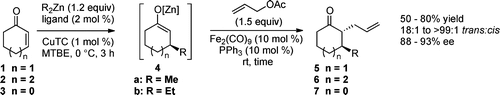
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2017

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
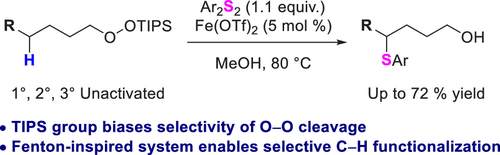
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
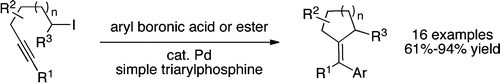
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
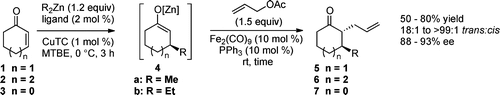
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2016

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
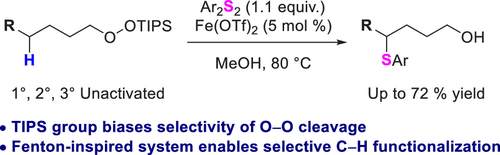
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
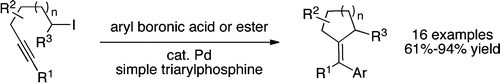
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
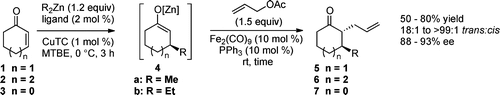
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2015

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
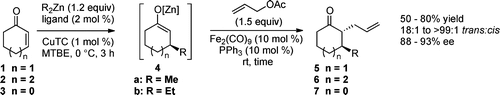
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2014

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
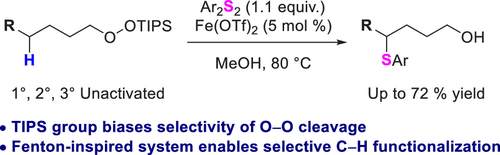
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2013

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
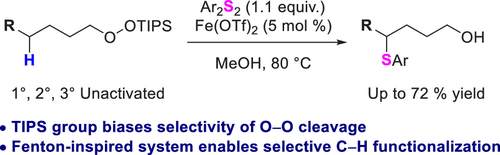
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
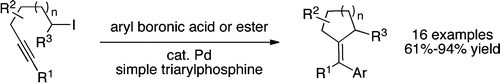
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
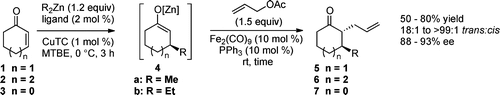
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2012

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
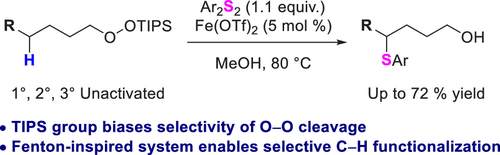
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
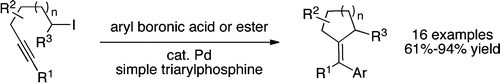
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
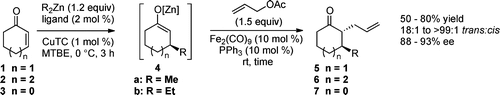
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

2011

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
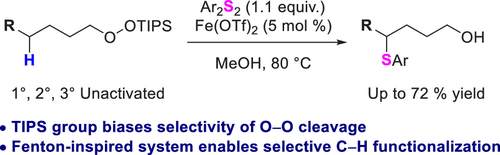
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
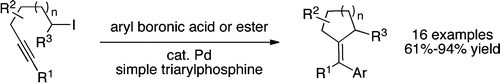
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
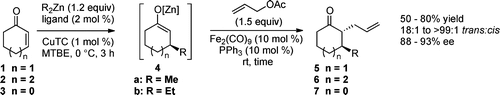
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

Pre IU Publications

de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
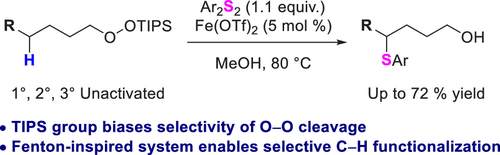
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication


de Gombert, A.; Darù, A.; Ahmed, T.; Haibach, M.; Li-Matsuura, R.; Yang, C.; Henry, R.; Cook, S. P.; Shekhar, S.; Blackmond, D. “Mechanistic Insight into Cu-Catalyzed C-N Coupling of Hindered Aryl Iodides and Anilines Using a Pyrrol-ol Ligand Enables Development of Mild and Homogeneous Reaction Conditions,” ACS Cat., 2023, 13(5), 2904–2915 (DOI: 10.1021/acscatal.0c02965).

Pinter, E. N.; Sheldon, Z. S.; Modak, A.; Cook, S. P. “Fluorosulfonamide-Directed Heteroarylation of Aliphatic C(sp3)–H Bonds,” J. Org. Chem., 2023, xx, xx-xx. [DOI: 10.1021/acs.joc.2c02461].

Lee, H.; He, T.; Cook, S. P. “Iron-Catalyzed, Directed Benzylic Borylation,” Org. Lett., 2023, 25(1), 1–4. [DOI: 10.1021/acs.orglett.2c02864].

Liu, Z. and Cook, S. P. “Directed Ni-Catalyzed Reductive Arylation of Aliphatic C–H Bonds,” Org. Lett., 2022, 24(18), 3313–3318. [DOI: 10.1021/acs.orglett.2c00447].

He, J.; Nguyen, T.; Guo, S.; Cook, S. P. “Csp3–H Trifluoromethylation of Unactivated Aliphatic Systems,” Org. Lett., 2021, 23(3), 702-705. [DOI: 10.1021/acs.orglett.0c03891].

Liu, Z. and Cook, S. P. “Interrupting the Barton-McCombie: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates,” Org. Lett., 2020, 23(3), 808–813. [DOI: 10.1021/acs.orglett.0c04039].

Modak, A.; Nett, A. J.; Swift, E. C.; Haibach, M. C.; Chan, V. S.; Franczyk, T. S. Shekhar, S.; Cook, S. P. “Cu-Catalyzed C-N Coupling with Sterically Hindered Partners,” ACS Cat., 2020, 141, 18405–18410 (DOI: 10.1021/acscatal.0c02965).
Highlights:
- Synfacts 2020, 16, 1434 (DOI: 10.1055/s-0040-1719552).

Pinter, E. N.; Bingham, J. E., AbuSalim, D. I., and Cook, S. P. “N-Directed Fluorination of Unactivated Csp3–H Bonds,” Chem. Sci., 2020, 11, 1102–1106 (10.1039/c9sc04055b).

Modak, A.; Pinter, E. N.; Cook, S. P. “Copper-Catalyzed, N-Directed Csp3–H Trifluromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3),” J. Am. Chem. Soc., 2019, 141, 18405–18410 (10.1021/jacs.9b10316).
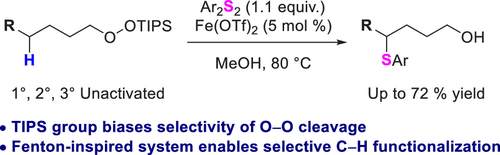
Groendyke, B.; Modak, A.; Cook, S. P. " Fenton-Inspired C–H Functionalization: Peroxide-Directed C–H Thioetherification," J. Org. Chem., 2019, 84, 13073–13091 (10.1021/jacs.9b101979).

Marcyk, P. T. and Cook, S. P. “Synthesis of Tetrahydroisoquinolines Through an Iron-Catalyzed Cascade: Tandem Alcohol Substitution and Hydroamination,”Org. Lett., 2019, 21, 6741–6744. [10.1002/acs.orglett.9b02353]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “1,2-(Bis)trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group,” Angew. Chem. Int. Ed., 2019, 58, 11704–11708. [10.1002/anie.201905247]

Sahota, N.; AbuSalim, D. I.; Wang, M. L.; Brown, C. J.; Zhang, Z.; El-Baba, T. J.; Cook, S. P.; Clemmer, D. E. “A microdroplet-accelerated Biginelli reaction: mechanisms and separation of isomers using IMS-MS,” Chem. Sci., 2019, 10, 4822–4827. [10.1039/C9SC00704K].

Marcyk, P. T. and Cook, S. P. “Iron-Catalyzed Hydroamination and Hydroetherification of Unactivated Alkenes,” Org. Lett., 2019, 21, 1547–1550. [10.1021/acs.orglett.9b00427]

Marcyk, P. T.; Jefferies, L. R.; AbuSalim, D. I.; Pink, M.; Baik, M.-H.; Cook, S. P. “Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides,”Angew. Chem. Int. Ed., 2019, 58, 1727–1731. [10.1002/anie.201812894]

Guo, S.; AbuSalim, D. I.; Cook, S. P. “Aqueous Benzylic Trifluoromethylation for Late-Stage Functionalization,” J. Am. Chem. Soc., 2018, 140, 12378–12382. [10.1021/jacs.8b08547] One of the "Most Read" papers in JACS for October!

Rosas Vargas, D.; Cook, S. P. “Palladium Nanoparticles: Chemoselective Control of the Reductive Heck with Aryl Triflates and 2,3-Dihydrofuran,” Tetrahedron, 2018, 74, 3314–3317. [10.1016/j.tet.2018.04.052] Invited Paper in honor of Professor Seth Herzon receiving the Tetrahedron Young Investigator Award.

Le Sueur, A. L.; Ramos, S.; Ellefsen, J. D.; Cook, S. P.; Thielges, M. C. “Evaluation of p-(13C,15N-Cyano)phenylalanine as an Extended Time Scale 2D IR Probe of Proteins,” Anal. Chem. 2017, 89, 5254–5260. [10.1021/acs.analchem.6b04650]

Groendyke, B.; AbuSalim, D. I.; Cook, S. P. “Iron-Catalyzed, Fluoroamide-Directed C–H Fluorination,” J. Am. Chem. Soc., 2016, 138, 12771–12774. [10.1021/jacs.6b08171] Highlighted in: C&E News 2016, 94, 40, October 10th. [link]

Atack, T. C. and Cook, S. P. “Manganese-Catalyzed Borylation of Unactivated Chlorides,” J. Am. Chem. Soc., 2016, 138, 6139–6142. [10.1021/jacs.6b03157] Highlighted in: ChemInform 2016, 47. [10.1002/chin.201646196]

Jefferies, L. R.; Weber, S. R.; Cook, S. P. “Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement,” Synlett, 2015, 26, 331–334. [10.1055/s-0034-1379540] Invited Paper for the "Catalysis with Sustainable Metals" Special Issue.

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Coupling of Aryl Sulfamates and Aryl/Vinyl Tosylates with Aryl Grignards," Org. Lett., 2014 16, 5080–5083 . [10.1021/ol024344]

Fruchey, E. R.; Monks, B. M.; Cook, S. P. "A Unified Strategy for Iron-Catalyzed ortho-Alkylation of Carboxamides," J. Am. Chem. Soc., 2014,136, 13130–13133. [10.1021/ja506823u]

Monks, B. M.; Fruchey, E. R.; Cook, S. P. "Iron-Catalyzed C(sp2)–H Alkylation of Carboxamides with Primary Electrophiles,"Angew. Chem. Int. Ed., 2014, 53, 11065–11069. [anie.201406594] Selected as "Hot Paper" by Angew. Chem.

Atack, T. C.; Lecker, R. M.; Cook, S. P. "Iron-Catalyzed Borylation of Alkyl Electrophiles," J. Am. Chem. Soc., 2014, 136, 9521–9523. [10.1021/ja505199u] Highlighted in: Org. Proc. Res. Dev. 2014, 18, 1047-1082. [10.1021/op500257q] Synfacts 2014, 10, 1070. [10.1055/s-0034-1379113] ChemInform 2015, 46, A. [10.1002/chin.201506218] Org. Chem. Highlights 2015, May 25. [link]

Jefferies, L. R.; Cook, S. P. “Alcohols as Electrophiles: Iron-Catalyzed Ritter Reactions and Benzyl Alcohol Additions to Alkynes,” Tetrahedron, 2014, 70, 4204–4207. [10.1016/j.tet.2014.03.072] Invited Paper in honor of Professor Sarah Reisman receiving the Tetrahedron Young Investigator Award.

Jefferies, L. R.; Cook, S. P. “Iron-Catalyzed Arene Alkylation Reactions with Unactivated Secondary Alcohols,” Org. Lett., 2014, 16, 2026–2029. [10.1021/ol500606d] Highlighted in: Synfacts 2014, 10, 747. [10.1055/s-0033-1339147] Org. Chem. Highlights 2014, October 13. [link]

Cook, S. P. “Artemisinin: A Case Study in the Evolution of Synthetic Strategy,” Synlett, 2014, 25, 751–759. [10.1055/s-0033-1340627] Invited Synpacts review.

Monks, B. M.; Cook, S. P. "Palladium-Catalyzed, Intramolecular Iodine-Transfer Reactions in the Presence of β-Hydrogens," Angew. Chem. Int. Ed., 2013, 52, 14214–14218. [anie.201308534]

Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Reduction Route to Trisubstituted Olefins," Org. Lett., 2013, 15, 4362–4365. [10.1021/ol4018694]

Cook, S. P. "The quest for affordable artemisinin," Future Med. Chem., 2013, 5, 233–236. [10.4155/fmc.13.1] Invited editorial.

DeLuca, R. J.; Edwards, J. L.; Steffens, L. D.; Michel, B. W.; Qiao, X.; Zhu, C.; Cook, S. P.; Sigman, M. S. "Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP," J. Org. Chem., 2013, 78, 1682–1686. [10.1021/jo302638v]

Agrawal, T.; Cook, S. P. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates," Org. Lett., 2013, 15, 96–99. [10.1021/ol303130j] Highlighted in: Org. Proc. Res. Dev. 2013, 17, 320-329. [10.1021/op400031g] Synfacts 2013, 9, 422. [10.1055/s-0032-1318378]
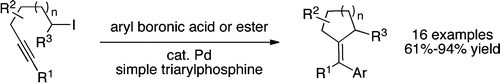
Monks, B. M.; Cook, S. P. "Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides," J. Am. Chem. Soc., 2012, 134, 15297–15300. [10.1021/ja3077611f]

Zhu, C.; Cook, S. P. "A Concise Synthesis of (+)-Artemisinin," J. Am. Chem. Soc., 2012, 134, 13577–13579. [10.1021/ja3061479] Highlighted in: One of 2012's "Most Read" papers in JACS. C&E News 2012, 90, 28. [link] JACS Spotlights 2012, 134, 15163–15164. [10.1021/ja3084187] Nature Chemical Biology 2012, 8, 808. [10.1038/nchembio.1073] "Synfact of the Month" Synfacts 2012, 11, 1163. [10.1055/s-0032-1317408] Nature Chemistry 2012, 4, 772. [10.1038/nchem.1473] "Synthesis: A Constructive Debate" Nature 2012, 492, 188. [10.1038/492188a] Org. Chem. Highlights 2013, April 15. [link]

Gao, P.; Cook, S. P. "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins," Org. Lett., 2012, 14, 3340–3343. [10.1021/ol3013167]

Jarugumilli, G. K.; Zhu, C.; Cook, S. P. "Re-Evaluating the Nucleophilicity of Zinc Enolates in Alkylation Reactions," Eur. J. Org. Chem., 2012, 1712–1715. [10.1002/ejoc.201200067]
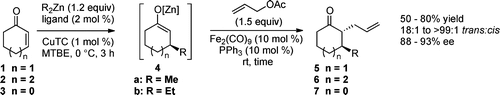
Jarugumilli, G. K.; Cook, S. P. "A Simple, Nontoxic Iron System for the Allylation of Zinc Enolates," Org. Lett., 2011, 13, 1904–1907. [10.1021/ol200059u]

This is a publication

Print Page